Safeguarding Pharmaceutical Integrity: Unveiling The Power Of Track & Trace Solutions
The pharmaceutical industry is a pillar of modern healthcare, providing medicines and vaccines that save lives and improve well-being. However, behind the scenes, a complex and challenging supply chain is at work, ensuring that pharmaceutical products reach patients in pristine condition, but this industry also faces numerous challenges. Biopharmaceutical waste amounts to a staggering $37 billion annually due to the complexities within cold chain logistics. These challenges encompass rapid temperature fluctuations during transit and in cold storage facilities, exposure to excessive light and heat, and even the risk of damage to protective packaging cases.
But what if there was a way to address these challenges effectively, ensuring pharmaceutical products' integrity and authenticity throughout the supply chain?
In this blog, we will delve into the world of pharmaceutical integrity and the critical role that track and trace solutions play in safeguarding it.
The Significance
of Pharma Product Integrity
The pharmaceutical industry has witnessed remarkable growth, with the global
biopharmaceutical and life sciences sector at a valuation of
$1,587.05 billion. However, this prosperity comes with challenges that can compromise the efficacy and safety of products.
The challenges faced by the pharmaceutical industry are multifaceted. Among the most pressing are rapid temperature fluctuations, exposure to excessive light and heat, and the potential for excessive shock exposure during transportation. These challenges often result in pharmaceutical waste amounting to a staggering $37 billion annually.
Counterfeiting, theft, and diversion are not just financial concerns but also pose significant health risks. We'll explore the consequences of these issues and highlight their potential to harm both patients and the industry as a whole.
Understanding Track and Trace Systems in Pharma
For combating these challenges, the pharmaceutical industry has turned to innovative track and trace solutions. These systems provide a digital thread that traces a product's journey from production to delivery.
Let's understand, in short, what a Track and Trace system means.
Track and trace is a comprehensive system that monitors and records the journey of pharmaceutical products from manufacturing facilities to patients' hands. This technology provides complete visibility over the supply chain, safeguarding product integrity and ensuring authenticity.
Types of Track and Trace Solutions Available for the Pharmaceutical Industry
We will explore the different types of track and trace solutions available, including serialisation, barcoding, and RFID technology.
1. Barcoding Systems:
Barcoding has been a foundational method of tracking products for decades. In pharmaceuticals, it involves unique identification through barcodes affixed to the packaging. These codes are scanned at different points in the supply chain, providing vital information about the product's origin, journey, and expiry.
2. Radio Frequency Identification (RFID):
RFID technology is an advanced method that uses radio waves to capture data stored on a tag or label attached to a product. In pharmaceuticals, RFID enables real-time tracking and monitoring, allowing for instant updates and the ability to access information without direct line-of-sight scanning.
3. Serialisation:
Serialisation is the process of assigning a unique serial number to individual units or batches of products, allowing each unit to be tracked and identified throughout its lifecycle. It enables pinpoint accuracy in tracing a product's path from manufacturing to distribution and beyond, fostering tighter control and authentication.
4. Data Matrix Codes:
These are 2D codes that store more information than traditional barcodes. Data matrix codes are utilised extensively in pharmaceutical packaging due to their compact size and capacity to contain vast amounts of data, enhancing traceability and compliance efforts.
5. Tamper-Evident Seals & Labels:
In a bid to ensure product safety and authenticity, tamper-evident seals and labels are employed to detect any unauthorised access or tampering. These labels break or indicate tampering, signalling that the product's integrity may have been compromised.
6. Blockchain Technology:
A more recent entrant in the field of track and trace, blockchain offers an immutable and decentralised ledger for tracking products. It promises enhanced security and transparency, enabling multiple stakeholders across the supply chain to access and authenticate data securely.
Each of these track and trace systems plays a vital role in ensuring product integrity, supply chain visibility, and compliance with stringent regulatory requirements. Pharmaceutical companies often combine multiple technologies to create robust and layered systems that significantly reduce the risk of counterfeiting, diversion, or tampering, ultimately safeguarding patient safety and industry reputation. An example of this kind of solution is what we offer at Adapt Ideations, you can check out them in detail from here.
These evolving technologies continuously shape the landscape of pharmaceutical track and trace systems, pushing the boundaries of what's achievable in ensuring product integrity.
Addressing Pharma Compliance & Supply Chain Security
Achieving Compliance & Security with Track and Trace
In a globalised world where pharmaceutical products traverse international boundaries, compliance with regulatory standards is non-negotiable. Different countries and organisations have set forth compliance standards to ensure that pharmaceutical products maintain their integrity throughout the supply chain.
One such example is the Australian Code of Good Wholesaling Practice for Medicines, which emphasises the need for monitoring temperature-sensitive medications during transport. Additionally, the FDA's Drug Supply Chain Security Act requires tracking of medicinal products from 2023 onward.
These laws underscore the pharmaceutical sector's shift towards ensuring end-to-end visibility throughout the global supply chain. The integration of track and trace solutions can not only ensure compliance but also strengthen data integrity and traceability, further securing the supply chain.
Read more about - Ensuring Supply Chain Integrity: Track and Trace Solutions for Pharmaceuticals.
Temperature-Controlled Transport & Pharma Product Integrity
Role of Temperature Monitoring in Ensuring Integrity
In the realm of pharmaceuticals, temperature-controlled transport is critical to maintaining product integrity. Many pharmaceutical products, such as medicines and vaccines, must be kept within a specific temperature range of 2°C to 8°C. Deviations from this range can render these products ineffective, jeopardising patient safety.
Temperature tracking and monitoring play a pivotal role in ensuring the integrity of pharmaceutical products. Rapid temperature fluctuations during transit can compromise the efficacy of these products. Exposure to excessive heat can lead to vaccine degradation, while cold storage facilities face the constant challenge of maintaining the required temperature range.
Streamlining Supply Chain Operations with Adapt Ideations’ Solutions
As we wrap up our exploration of pharmaceutical integrity, we present a solution that can revolutionise your supply chain operations.
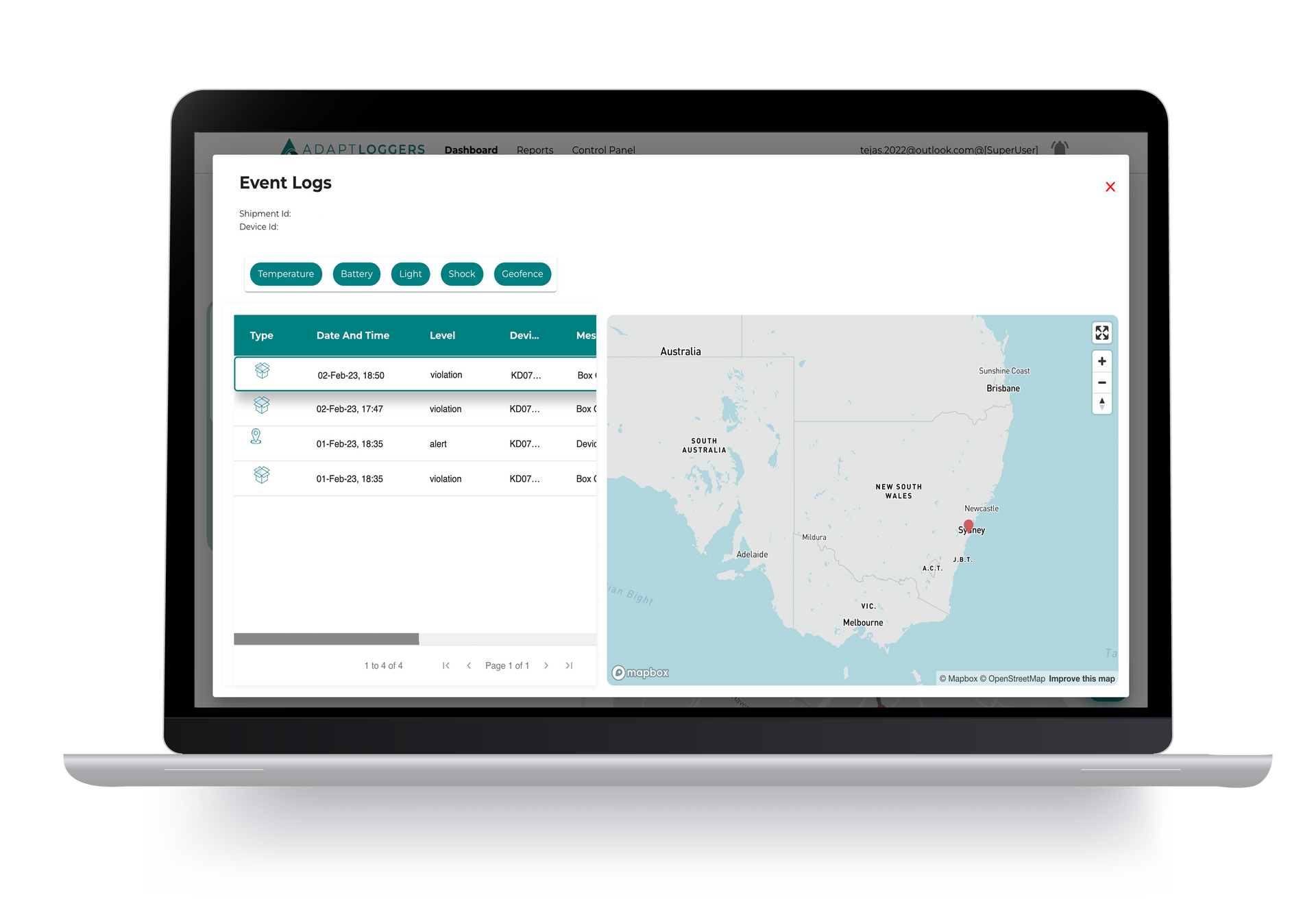
Adapt Ideations' KELVIN IoT Monitoring Track and Trace Devices offer real-time visibility and monitoring of temperature-sensitive shipments, ensuring product integrity throughout the supply chain.
For pharmaceutical cold storage spaces, the PIXEL temperature data loggers from Adapt Ideations provide real-time visibility on temperature and humidity conditions, helping you achieve efficiency while maintaining compliance with pharmaceutical standards.
The innovative devices enable real-time monitoring and immediate alerts, enabling quick responses to deviations of set conditions, ultimately enhancing your operations.
In A Nutshell
The pharmaceutical industry is at a crossroads where we must maintain authenticity and integrity to ensure patient safety. Track and trace solutions are the linchpin in achieving this goal.
We at
Adapt Ideations offer a practical and innovative solution to these challenges. Our
KELVIN &
PIXEL IoT Monitoring Track and Trace Devices
ensure real-time monitoring and visibility, helping improve efficiency, and compliance with pharmaceutical standards. With sustainability at the core of our values, we're committed to promoting greener cold chains and making a positive impact on the environment.
Don't let temperature-sensitive pharmaceutical products in your supply chain go unprotected. Take a step towards enhancing your product integrity and ensuring patient safety. Reach out to Adapt Ideations and embark on a journey towards a greener, safer, and more efficient pharmaceutical supply chain.
Contact us today, and let's redefine the future of pharmaceutical integrity together.
Enquire to find out more about our innovative solutions and how they can be utilised at enquiries@adaptideations.com
Share Our Post.
Awards & Recognition

Best Temperature Monitoring Solution Provider
Awarded by India Biologics & Vaccines Outstanding Industry Awards 2022

Adapt Ideations Recognised As A Supply Chain Leader
by Alcott Global on Supplify's Supply Chain Tech Map 2.0

Related Articles.